Multiple Choice
The reaction below is a direct enolate alkylation.It has been found that this reaction only works well with unhindered methyl and 1° alkyl halides.Pick the statement that best explains this observation. 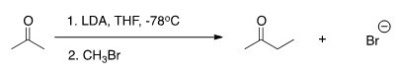
A) The nucleophilic enolate requires a reaction center that has a positive charge.
B) Hindered alkyl halides do not undergo SN1 reactions.
C) Hindered alkyl halides do not undergo SN2 reactions.
D) Methyl and 1° alkyl halides can form carbocations that can readily react with the nucleophilic enolate.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Which of the following ketones will give
Q4: Why is it difficult to stop the
Q5: What are the three steps in the
Q6: What is the structure of X,product of
Q7: Will acetophenone be completely deprotonated by lithium
Q9: Which is the most acidic proton in
Q10: Select the appropriate sequence of reactions to
Q11: Which of the following is an enol
Q12: Which is the kinetic enolate of 2-methylcyclohexanone?
Q13: Which of the following are enol forms