Multiple Choice
Treatment of 2-hexanone with NaOCH2CH3 followed by CH3Br affords compound X (C7H14O) as the major product.X shows a strong absorption in the IR spectrum at 1713 cm-1,and its 1H NMR data is given below.What is the structure of X? 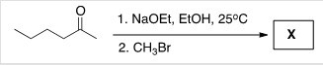

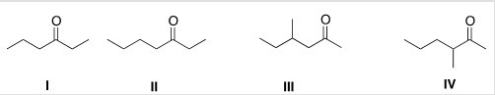
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Q26: Which of the following is the intermediate
Q27: Which is the more stable form of
Q28: What is the missing reagent for the
Q29: Vitamin C is a stable enediol.Which is
Q30: Which of the following compounds is an
Q32: Starting with cyclohexanone,how could you prepare the
Q33: What is (are)the product(s)of the following reaction?
Q34: Which of the following would undergo racemization
Q35: What is the starting material in the
Q36: The following molecule is called <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7662/.jpg"