Multiple Choice
How would the following compounds be distinguishable using IR and 1H NMR spectroscopy? 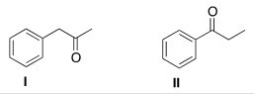
A) The 1H NMR spectrum of compound I will have two singlets.
B) The C=O absorption in the IR spectrum of compound I will be at a higher wave number than that of compound II.
C) The 1H NMR spectrum of compound II will have one triplet at a chemical shift of about 4.
D) The 1H NMR spectrum of compound I will have two singlets AND the C=O absorption in the IR spectrum of compound I will be at a higher wave number than that of compound II.
Correct Answer:

Verified
Correct Answer:
Verified
Q31: What is the product of the following
Q32: What is the product of the following
Q33: What is the product of the following
Q34: Which of the following statements about carbohydrates
Q35: What is the product of the following
Q37: Identify how you could synthesize an enamine.<br>A)React
Q38: Name the following aldehyde. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7662/.jpg" alt="Name
Q39: Using IR spectroscopy,how can you tell the
Q40: Which is the most reactive carbonyl compound?
Q41: What product is formed when the following