Multiple Choice
Which of the following statements about the molecular orbital (MO) theory is true? 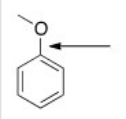
A) When two p orbitals of similar phase overlap side-by-side,a p* antibonding molecular orbital is formed.
B) When two p orbitals of opposite phase overlap side-by-side,a p bonding molecular orbital is formed.
C) A p bonding molecular orbital is higher in energy than the two atomic p orbitals from which it is formed.
D) A p* antibonding molecular orbital is higher in energy than the two atomic p orbitals from which it is formed.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: In what type of orbital does the
Q7: What is the correct structure for anisole?
Q8: What is the correct structure for aniline?
Q9: What is the IUPAC name of the
Q10: What compound is consistent with the following
Q12: What is the IUPAC name of the
Q13: What orbitals are used to form the
Q14: How many <sup>13</sup>CNMR signals does the following
Q15: What is the name of the following
Q16: Which of the following molecules has the