Multiple Choice
Using the bond dissociation energies given,calculate DH° for the following reaction. 
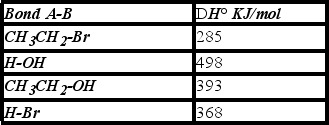
A) +108 KJ/mol
B) -130 KJ/mol
C) -22 KJ/mol
D) +22 KJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: Which of the following statements about bond
Q16: Which of the following statements about substitution
Q17: The conversion of acetyl chloride to methyl
Q18: What kind of reaction does the conversion
Q19: Which of the following statements about equilibrium
Q21: What type of bond cleavage takes place
Q22: Which of the following statements is not
Q23: The conversion of acetyl chloride to methyl
Q24: The symbol Δ stands for _ in
Q25: Which of the following statements is not