Multiple Choice
Which of the following pair does not represent resonance structures? 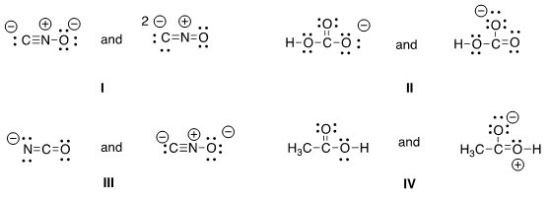
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q49: When forming molecular orbitals from atomic orbitals,what
Q50: Which of the following is the appropriate
Q51: Which of the following would most likely
Q52: How many electrons are around phosphorus in
Q53: Determine the geometry around the indicated atom
Q55: Which atomic orbitals overlap to form the
Q56: What is the approximate value of the
Q57: How many constitutional isomers are there for
Q58: What is the approximate bond angle for
Q59: What is the molecular geometry around the