Multiple Choice
Using the laws of constant composition and the conservation of mass,complete the molecular picture of hydrogen molecules (circles) reacting with oxygen molecules (squares) to give water. 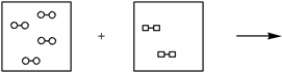
A) 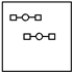
B) 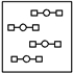
C) 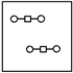
D) 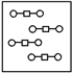
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Q5: What is the correct name for K<sub>3</sub>PO<sub>4</sub>?<br>A)
Q6: F-20,a radioactive isotope of fluorine,has<br>A) 9 protons,10
Q7: What is the correct name for PF<sub>5</sub>?<br>A)
Q9: What is the correct name for Al<sub>2</sub>O<sub>3</sub>?<br>A)
Q11: Which three elements are likely to have
Q12: How many nonmetallic elements are there in
Q13: Silver has two stable isotopes with masses
Q14: Which two atoms below have the
Q15: Which species has 63 neutrons?<br>A)
Q17: What is the correct name for CCl<sub>4</sub>?<br>A)