Multiple Choice
Using the laws of constant composition and the conservation of mass,complete the molecular picture of hydrogen molecules (circles) reacting with chlorine molecules (squares) to give hydrogen chloride (HCl) . 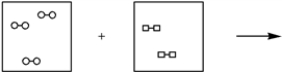
A) 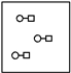
B) 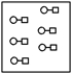
C) 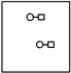
D) 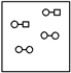
E) None of these are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Which of the following atoms contains the
Q45: What is the correct formula for sulfur
Q46: What is the identity of
Q47: What is the correct name for N<sub>2</sub>O<sub>3</sub>?<br>A)
Q48: Group 1 elements are also known as<br>A)
Q52: What is the correct formula for aluminum
Q53: Which of the following is a nonelectrolyte
Q54: The average molar mass of lithium is
Q55: Which element is most likely to form
Q56: Sodium sulfate has the chemical formula Na<sub>2</sub>SO<sub>4</sub>.Based