Multiple Choice
Would this crossed Aldol reaction work well? Why or why not? 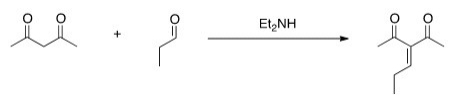
A) Yes,the diketone is significantly more acidic,so this enolate can be formed selectively.
B) Yes,the aldehyde is significantly more acidic,so this enolate can be formed selectively.
C) No,the aldehyde is significantly more acidic,so this enolate cannot be formed selectively.
D) No,the diketone is significantly more acidic,so this enolate cannot be formed selectively.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: What is the product of the self-condensation
Q7: Which of the following compounds can undergo
Q8: What product (including stereochemistry)is formed in the
Q9: What is the product of the following
Q12: What cyclic product is formed in the
Q14: What type of esters can undergo Claisen
Q15: Under basic conditions,the Aldol reaction is reversible,but
Q15: What is the product of the following
Q17: In a Michael reaction,where does the nucleophile
Q26: When is a crossed Claisen reaction between