Multiple Choice
Why can't you prepare 2-tert-butylcyclohexanone by the following reaction? 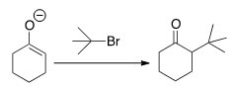
A) Because tert-butyl bromide is too basic.
B) Because tert-butyl bromide cannot undergo an SN2 reaction.
C) Because tert-butyl bromide is a nucleophile.
D) Because tert-butyl bromide is not a stable compound.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: If you want to form a kinetic
Q2: It has been found that b-dicarbonyl compounds
Q17: For most compounds with a single keto
Q31: Which is the most acidic proton in
Q32: What is the missing reagent for the
Q37: Which of the following compounds would undergo
Q38: What is the missing reagent in the
Q40: Which of the following is an enol
Q41: Why is the enolate of acetone less
Q41: Which of the following are enol forms