Multiple Choice
Starting with cyclohexanone,how could you prepare the diketone below? 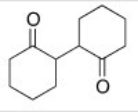
A) Treat cyclohexanone with a base under thermodynamic conditions.
B) Hydrogenate cyclohexanone with Raney nickel.
C) Convert cyclohexanone into the a-bromoketone and then react this with the enolate of cyclohexanone.
D) Convert cyclohexanone into an enamine with diethylamine and then react this with more cyclohexanone.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: If you want to form a kinetic
Q2: It has been found that b-dicarbonyl compounds
Q7: Will acetophenone be completely deprotonated by lithium
Q23: Which of the following compounds is the
Q24: What is the starting material for the
Q26: Which of the following is the least
Q30: Which of the following four compounds is
Q31: Which is the most acidic proton in
Q32: What is the missing reagent for the
Q40: Which of the following bases will completely