Multiple Choice
Why would the alcohol in the following compound need to be protected before reaction? 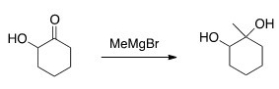
A) If it isn't protected,the product will be a carboxylic acid.
B) The Grignard reagent will react with the alcohol before the ketone.
C) Magnesium is Lewis acidic and will coordinate with the alcohol.
D) There is no need to protect the alcohol.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: What reagent can be used to cleave
Q26: Which of the following statements about organometallic
Q38: What is the missing reagent in the
Q40: What is the product of the following
Q41: What is the missing reagent in the
Q42: Rank the carbon-metal bond in the following
Q44: What is the major organic product of
Q46: What is the major organic product of
Q47: What is the major product from the
Q49: If a compound is reduced,what is the