Multiple Choice
The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism: 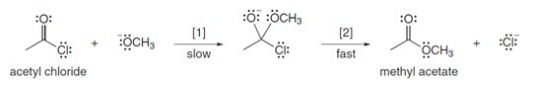 If the concentrations of both -OCH3 and acetyl chloride were increased 2 times,what would happen to the rate of the reaction?
If the concentrations of both -OCH3 and acetyl chloride were increased 2 times,what would happen to the rate of the reaction?
A) Rate would become one-fourth
B) Rate would increase 4 times
C) Rate would increase 16 times
D) Rate would increase 2 times
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Which of the following statements about enzymes
Q15: Which of the following statements about bond
Q20: What kind of reaction does the conversion
Q22: Which of the following statements is not
Q23: The equilibrium constant for the conversion of
Q24: The conversion of acetyl chloride to methyl
Q26: Which of the following statements is not
Q27: Which of the K<sub>eq</sub> corresponds to the
Q29: What is the name given to the
Q30: Which of the following letters represents DH°