Multiple Choice
Determine the geometry around the indicated atom in each species. 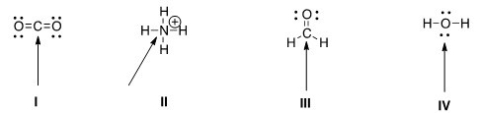
A) I = Linear; II = tetrahedral; III = trigonal planar; IV = tetrahedral
B) I = Linear; II = tetrahedral; III = trigonal planar; IV = linear
C) I = Trigonal planar; II = linear; III = tetrahedral; IV = trigonal planar
D) I = Tetrahedral; II = trigonal planar; III = linear; IV = tetrahedral
Correct Answer:

Verified
Correct Answer:
Verified
Q27: Which of the following would you expect
Q38: Which of the following compounds has an
Q43: What is the hybridization of the nitrogen
Q48: Which of the following molecules has non-polar
Q57: How many constitutional isomers are there for
Q64: Which of the following Lewis structures is
Q68: Which of the following is the appropriate
Q69: Which atomic orbitals overlap to form the
Q70: Which compound contains the most polar bond?
Q72: Which of the following is the appropriate