Multiple Choice
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon-oxygen double bond. Calculate the enthalpy of reaction using the bond energies given. 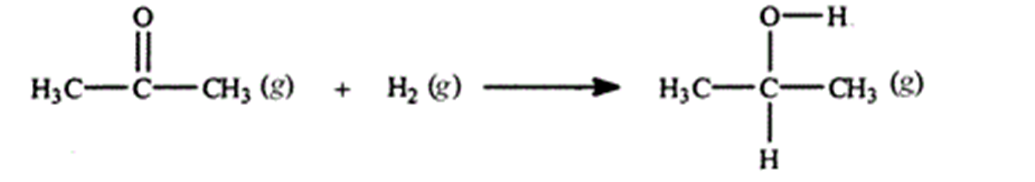
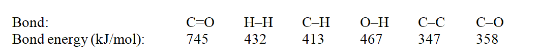
A) -484 kJ
B) -366 kJ
C) -61 kJ
D) +61 kJ
E) +366 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The lattice energy of large ions is
Q5: In covalent bond formation, the potential energy
Q18: Which one of the following properties is
Q19: The lattice energy for ionic crystals increases
Q19: Select the element whose Lewis symbol is
Q20: Calculate the lattice energy of magnesium sulfide
Q25: Which of the following elements is the
Q32: Arrange the following bonds in order of
Q46: In which of the following processes does
Q56: The majority of elements are good electrical