Multiple Choice
Sodium chlorate is used as an oxidizer in the manufacture of dyes, explosives, and matches. Calculate the mass of solute needed to prepare 1.575 L of 0.00250 M NaClO3 ( 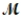 = 106.45 g/mol) .
= 106.45 g/mol) .
A) 419 g
B) 169 g
C) 0.419 g
D) 0.169 g
E) 0.00394 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: How many moles of H<sup>+</sup>(aq) ions are
Q17: Calculate the oxidation number of the chlorine
Q27: Select the correct name and chemical formula
Q30: A particular reaction may be both a
Q59: Predict the product(s) for the following reaction.
Q73: What will be the final volume of
Q82: Select the classification for the following reaction.
Q86: How many milliliters of 1.58 M HCl
Q91: Ammonia, NH<sub>3</sub>, produces hydroxide ions in aqueous
Q98: Select the net ionic equation for the