Multiple Choice
Calculate the molarity of a 23.55-mL solution which contains 28.24 mg of sodium sulfate (used in dyeing and printing textiles, 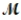 = 139.04 g/mol) .
= 139.04 g/mol) .
A) 8.625 M
B) 1.199 M
C) 0.8339 M
D) 0.2031 M
E) 0.008625 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: Potassium carbonate, K<sub>2</sub>CO<sub>3</sub>, sodium iodide, NaI, potassium
Q21: How many moles of H+(aq) ions are
Q22: Which of the following is most soluble
Q23: Hydrochloric acid is widely used as a
Q24: How many moles of ions are released
Q25: Which one of the following is not
Q31: Sodium hydroxide, also known as caustic soda,
Q53: Which one of the following substances, when
Q74: Which one of the following substances is
Q85: Potassium chloride, KCl, sodium sulfate, Na<sub>2</sub>SO<sub>4</sub>, glucose,