Multiple Choice
Magnesium reacts with iron(III) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron. 3Mg(s) + 2FeCl3(s) → 3MgCl2(s) + 2Fe(s)
A mixture of 41.0 g of magnesium ( 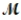 = 24.31 g/mol) and 175 g of iron(III) chloride (
= 24.31 g/mol) and 175 g of iron(III) chloride ( 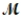 = 162.2 g/mol) is allowed to react. What mass of magnesium chloride = 95.21 g/mol) is formed?
= 162.2 g/mol) is allowed to react. What mass of magnesium chloride = 95.21 g/mol) is formed?
A) 68.5 g MgCl2
B) 77.0 g MgCl2
C) 71.4 g MgCl2
D) 107 g MgCl2
E) 154 g MgCl2
Correct Answer:

Verified
Correct Answer:
Verified
Q7: In combustion analysis, the oxygen content of
Q7: What is the mass in grams of
Q10: Calculate the molar mass of rubidium carbonate,
Q15: Terephthalic acid, used in the production of
Q16: The number of hydrogen atoms in 0.050
Q22: In combustion analysis, the carbon and hydrogen
Q27: Methanol (CH<sub>4</sub>O) is converted to bromomethane (CH<sub>3</sub>Br)
Q47: Terephthalic acid, used in the production of
Q49: Hydroxylamine nitrate contains 29.17 mass % N,
Q63: Calculate the molar mass of (NH<sub>4</sub>)<sub>3</sub>AsO<sub>4</sub>.<br>A)417.80 g/mol<br>B)193.03