Multiple Choice
What is the percent yield for the reaction PCl3(g) + Cl2(g) → PCl5(g)
If 119.3 g of PCl5 (M = 208.2 g/mol) are formed when 61.3 g of Cl2 ( 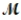 = 70.91 g/mol) react with excess PCl3?
= 70.91 g/mol) react with excess PCl3?
A) 195%
B) 85.0%
C) 66.3%
D) 51.4%
E) 43.7%
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Ammonia, an important source of fixed nitrogen
Q9: A normal breath takes in about 1.0
Q11: Balance the following equation: B<sub>2</sub>O<sub>3</sub>(s) + HF(l)
Q28: Phosphorus pentachloride, PCl<sub>5</sub>, a white solid that
Q33: Tetraphosphorus hexaoxide ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="Tetraphosphorus hexaoxide
Q39: Household sugar, sucrose, has the molecular formula
Q42: Sulfur dioxide reacts with chlorine to produce
Q56: Phosphine, an extremely poisonous and highly reactive
Q65: Which of the following samples has the
Q70: Aluminum metal reacts with chlorine gas to