Multiple Choice
The isotopes of promethium, 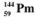 and
and 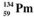 are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
A) promethium-144, β decay; promethium-134, positron decay
B) promethium-144, positron decay; promethium-134, β decay
C) promethium-144, positron decay; promethium-134, electron capture
D) promethium-144, electron capture; promethium-134, positron decay
E) promethium-144, β decay; promethium-134, 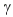 decay
decay
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which one of the following equations correctly
Q3: Which one of the following equations correctly
Q5: An alkaline earth element is radioactive. It
Q7: The radioisotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="The radioisotope
Q10: Select the nuclide that completes the following
Q11: The isotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="The isotope
Q13: Gamma rays are high energy electrons.
Q21: Cesium-134 is a β emitter with a
Q71: Most foodstuffs contain natural, radioactive isotopes.
Q76: No alpha decay is observed for isotopes