Multiple Choice
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 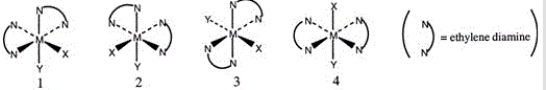 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?
A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.
Correct Answer:

Verified
Correct Answer:
Verified
Q34: A characteristic of ligands is that<br>A)they are
Q36: The Cu<sup>2+</sup> ion has 1 unpaired electron.
Q54: Octahedral complexes can exhibit geometric, optical, and
Q56: The ground state electronic configuration of Zn<sup>2+</sup>
Q61: Which one of the following normally acts
Q63: In the presence of a strong octahedral
Q64: Which of the following ions is least
Q71: What is the highest possible oxidation state
Q76: In the formation of a transition metal
Q78: Write the formula for sodium tetracyanonickelate(II).<br>A)Na[Ni(CN)<sub>4</sub>]<br>B)Na[Ni(CN)<sub>4</sub>] <sub>2</sub><br>C)Na