Multiple Choice
Consider the following structures (1 and 2 are octahedral; 3 and 4 are square planar) . 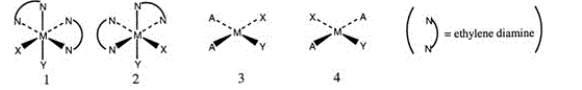 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?
A) 1 and 2 are superimposable.
B) 1 and 2 are geometric isomers.
C) 3 and 4 are structural isomers.
D) 3 and 4 are optical isomers.
E) 3 and 4 are geometric isomers.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: The permanganate ion (MnO<sub>4</sub><sup>−</sup>) is a powerful
Q12: How many unpaired electrons will there be
Q17: A certain transition metal complex has the
Q19: When the ethylenediaminetetraacetate ion (EDTA<sup>4-</sup>) forms a
Q21: Which of the following ions could exist
Q27: The ground state electron configuration of a
Q35: Which of the following ligands is most
Q38: The most common oxidation state for ions
Q47: How many unpaired electrons are there in
Q59: Which of the following ions could exist