Multiple Choice
The temperature at which the following process reaches equilibrium at 1.0 atm is the normal melting point for phosphoric acid. H3PO4(s)  H3PO4(l)
H3PO4(l)
Use the following thermodynamic information at 298 K to determine this temperature.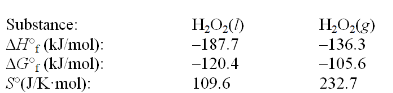
A) 286 K
B) 305 K
C) 315 K
D) 347 K
E) 3170 K
Correct Answer:

Verified
Correct Answer:
Verified
Q5: A reaction has ΔG = 10.0 kJ
Q6: Elemental boron can be formed by reaction
Q9: Given: H<sub>2</sub>O(l) → H<sub>2</sub>O(s) ΔH° = −6.02
Q11: Calculate ΔG° for the combustion of propane.
Q21: Which relationship or statement best describes ΔS°
Q23: Which relationship or statement best describes ΔS°
Q33: Under a given set of conditions, all
Q34: Which relationship or statement best describes ΔS°
Q64: For a chemical reaction to be spontaneous
Q80: Which relationship or statement best describes ΔS°