Multiple Choice
Consider the figure that shows ΔG° for a chemical process plotted against absolute temperature. 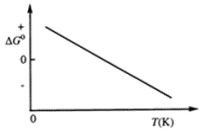 Which one of the following is an incorrect conclusion, based on the information in the diagram?
Which one of the following is an incorrect conclusion, based on the information in the diagram?
A) ΔH° > 0
B) ΔS° > 0
C) The reaction is spontaneous at high temperatures.
D) ΔS° increases with temperature while ΔH° remains constant.
E) There exists a certain temperature at which ΔH° = TΔS°.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Which one of the following phase changes
Q20: Which of the following values is based
Q29: Elemental boron can be formed by reaction
Q31: Which relationship or statement best describes ΔS°
Q35: Calculate ΔG° for the reaction SiCl<sub>4</sub>(g) +
Q35: Which relationship or statement best describes ΔS°
Q38: The temperature at which the following process
Q40: For any reaction, if ΔG° > 0,
Q48: Which of the following pairs has the
Q74: Which of the following results in a