Multiple Choice
Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen. The data refer to 25°C. CH4(g) + 2H2O(g)  CO2(g) + 4H2(g)
CO2(g) + 4H2(g) 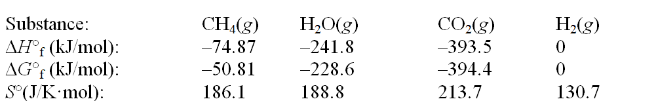
A) 8.2 × 1019
B) 0.96
C) 0.58
D) 1.2 × 10-20
E) 1.4 × 10-46
Correct Answer:

Verified
Correct Answer:
Verified
Q1: For a chemical reaction to be spontaneous
Q11: You are given pure samples of ammonia,
Q22: Consider the reaction CuI(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="Consider
Q25: The free energy of a perfect crystal
Q25: In 1774 Joseph Priestley prepared the element
Q26: Which relationship best describes ΔS° for the
Q29: Elemental boron can be formed by reaction
Q55: In order for a process to be
Q63: Which, if any, of the following processes
Q66: Which of the following is true for