Multiple Choice
Silicon, which makes up about 25% of Earth's crust by mass, is used widely in the modern electronics industry. It has three naturally occurring isotopes, 28Si, 29Si, and 30Si. Calculate the atomic mass of silicon. 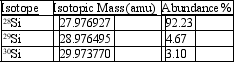
A) 29.2252 amu
B) 28.9757 amu
C) 28.7260 amu
D) 28.0855 amu
E) 27.9801 amu
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Which of the following ions occurs commonly?<br>A)P
Q38: Who is credited with measuring the mass/charge
Q41: The mass of a neutron is equal
Q44: The compound, NaH<sub>2</sub>PO<sub>4</sub>, is present in many
Q45: Atoms X, Y, Z, and R have
Q51: What is the name of the acid
Q52: What is the name of Na<sub>2</sub>O?<br>A) disodium
Q64: Copper (Cu) is a transition metal.
Q68: Zinc acetate is used in preserving wood
Q73: Silver chloride is used in photographic emulsions.