Multiple Choice
Which one of the following is the best representation of the titration curve that will be obtained in the titration of a weak acid (0.10 mol L-1) with a strong base of the same concentration? 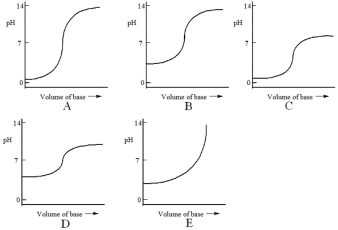
A) A
B) B
C) C
D) D
E) E
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Calculate the solubility of silver chromate, Ag<sub>2</sub>CrO<sub>4</sub>,
Q31: Calculate the solubility of barium carbonate, BaCO<sub>3</sub>,
Q32: A lab technician adds 0.015 mol of
Q33: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q35: The solubility of magnesium phosphate is 2.27
Q36: Which of the following indicators would be
Q37: What is the [H<sub>3</sub>O<sup>+</sup>] in a buffer
Q38: A solution is prepared by mixing 50.0
Q39: A 20.0-mL sample of 0.30 M HClO
Q51: For a diprotic acid H<sub>2</sub>A, the relationship