Multiple Choice
Write the ion product expression for magnesium fluoride, MgF2.
A) 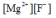
B) 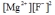
C) 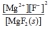
D) 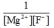
E) 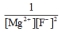
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: What volume of 0.500 M H<sub>2</sub>SO<sub>4 </sub>is
Q39: A buffer is to be prepared by
Q64: When 20.0 mL of 0.15 M hydrochloric
Q72: The solubility of silver chloride _ when
Q83: Calculate the solubility of lead(II) iodide, PbI<sub>2</sub>,
Q84: Which of the following substances has the
Q86: If 10.0 g of NaF and 20.0
Q89: The solubility of lead(II) chloride is 0.45
Q91: A buffer is prepared by adding 1.00
Q106: When a weak acid is titrated with