Multiple Choice
Write the mass-action expression, 
c, for the following chemical reaction equation.
2C6H6(g) + 15O2(g)  12CO2(g) + 6H2O(g)
12CO2(g) + 6H2O(g)
A) 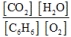
B) 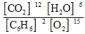
C) 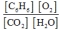
D) 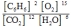
E) 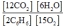
Correct Answer:

Verified
Correct Answer:
Verified
Q11: If all of the coefficients in the
Q21: A chemical reaction has an equilibrium constant
Q43: H<sub>2</sub>SO<sub>3</sub>(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="H<sub>2</sub>SO<sub>3</sub>(aq) HSO<sub>3</sub>(aq)
Q44: Consider the following two equilibria and their
Q45: A mixture of 0.500 mole of carbon
Q47: Which of the following has an effect
Q50: Sodium hydrogen carbonate decomposes above 110°C to
Q51: What is the mass-action expression, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg"
Q53: The following reaction is at equilibrium in
Q79: Chemical reactions generally reach equilibrium because one