Multiple Choice
Write the mass-action expression,  c, for the following chemical reaction. Fe3+(aq) + 3OH-(aq)
c, for the following chemical reaction. Fe3+(aq) + 3OH-(aq)  Fe(OH) 3(s)
Fe(OH) 3(s)
A) 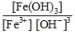
B) 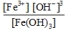
C) 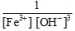
D) 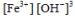
E) 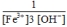
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: The reaction of nitric oxide to form
Q8: Hydrogen bromide will dissociate into hydrogen and
Q10: The equilibrium constant, Kp, for the reaction
Q11: Consider the equilibrium reaction: H<sub>2</sub>(g) + Br<sub>2</sub>(g)
Q12: Consider the equilibrium reaction shown below. B2(g)
Q14: Carbon monoxide and chlorine combine in an
Q31: A good catalyst for a reaction will
Q53: When a reaction system reaches equilibrium, the
Q63: Once a reaction system reaches equilibrium, the
Q78: If all the reactants and products in