Multiple Choice
Boron has 3 valence electrons. Which of the following processes is involved in boron's achieving a complete outer shell?
A) formation of a B3+ cation
B) formation of bridge bonds
C) formation of 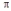 -bonds using its d-orbitals
-bonds using its d-orbitals
D) formation of 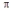 -bonds using sp3-orbitals
-bonds using sp3-orbitals
E) None of these choices are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Bromine will form compounds with each of
Q18: Predict the products for the reaction of
Q52: Oxides of group 1A (1) and 2A
Q55: Comparing neon with xenon, one would expect
Q60: Which of the following will have the
Q61: The acidity of oxides of main group
Q74: Which of the following oxides is most
Q97: Hydrogen fluoride is used<br>A)in the manufacture of
Q99: Although the periodic table is organized according
Q100: Which of the following hydroxides will be