Multiple Choice
Which of the following statements describes the correct method of preparation of 1.00 L of a 2.0 M urea solution? 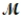 urea = 60.06 g/mol
urea = 60.06 g/mol
A) Dissolve 120 g of urea in 1.00 kg of distilled water.
B) Dissolve 120 g of urea in 880 g of distilled water.
C) Dissolve 120 g of urea in enough distilled water to produce 1.00 L of solution.
D) Dissolve 120 g of urea in 1.00 liter of distilled water.
E) The density of urea is needed in order to do this calculation.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Procaine hydrochloride ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="Procaine hydrochloride
Q23: The Tyndall effect<br>A)is observed in concentrated solutions.<br>B)is
Q24: Two aqueous are prepared: 1.00 m Na<sub>2</sub>CO<sub>3</sub>
Q41: If the density of a solution is
Q44: How many moles of sulfate ions are
Q46: Dimethylglyoxime, DMG, is an organic compound used
Q48: What concentration of aqueous FeCl<sub>3</sub> would have
Q51: Electrolyte solutions generally behave less ideally as
Q85: A saturated solution of carbon dioxide in
Q86: What is the mole fraction of Ne