Multiple Choice
Calculate the vapor pressure of a solution prepared by dissolving 0.500 mol of a non-volatile solute in 275 g of hexane ( 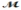 = 86.18 g/mol) at 49.6°C. P°hexane = 400.0 torr at 49.6°C.
= 86.18 g/mol) at 49.6°C. P°hexane = 400.0 torr at 49.6°C.
A) 54 torr
B) 154 torr
C) 246 torr
D) 346 torr
E) 400. torr
Correct Answer:

Verified
Correct Answer:
Verified
Q4: An emulsion is a dispersion consisting of
Q9: Raoult's Law relates the vapor pressure of
Q12: Select the weakest electrolyte from the following
Q25: The mole fraction of potassium nitrate in
Q37: Potassium fluoride is used for frosting glass.
Q52: Colligative properties depend on<br>A)the chemical properties of
Q65: Benzaldehyde ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="Benzaldehyde (
Q74: The solubility of gases in water increases
Q78: Heats of solution may be either positive
Q79: Calculate the molarity of a solution prepared