Multiple Choice
Safrole is used as a topical antiseptic. Calculate the vapor pressure of a solution prepared by dissolving 0.75 mol of safrole in 950 g of ethanol ( 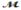 = 46.07 g/mol) . P°ethanol = 50.0 torr at 25°C.
= 46.07 g/mol) . P°ethanol = 50.0 torr at 25°C.
A) 1.8 torr
B) 11 torr
C) 15 torr
D) 40 torr
E) 48 torr
Correct Answer:

Verified
Correct Answer:
Verified
Q6: A 0.100 m K<sub>2</sub>SO<sub>4</sub> solution has a
Q21: What volume of concentrated (14.7 M) phosphoric
Q30: Raoult's Law relates the vapor pressure of
Q42: Consider the expression below, showing the terms
Q45: Saccharin, one of the first non-nutritive sweeteners
Q50: The heat of solution is the total
Q52: The concentration of iodine in sea water
Q60: Two aqueous solutions are prepared: 2.0 m
Q68: Copper(II) bromide is used as a wood
Q89: The chemist, Anna Lytic, must prepare 1.00