Multiple Choice
Carbon tetrachloride, once widely used in fire extinguishers and as a dry cleaning fluid, has been found to cause liver damage to those exposed to its vapors over long periods of time. What is the boiling point of a solution prepared by dissolving 375 g of sulfur (S8, 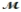 = 256.5 g/mol) in 1250 g of CCl4? Kb = 5.05°C/m, boiling point of pure CCl4 = 76.7°C?
= 256.5 g/mol) in 1250 g of CCl4? Kb = 5.05°C/m, boiling point of pure CCl4 = 76.7°C?
A) 70.8°C
B) 75.2°C
C) 78.2°C
D) 82.6°C
E) >85°C
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Which of the following sets of conditions
Q26: Barbiturates are synthetic drugs used as sedatives
Q27: Calculate the freezing point of a solution
Q28: Under physiological conditions, amino acids in solution
Q29: A 0.100 m MgSO4 solution has a
Q34: Which of the following aqueous solutions will
Q35: Which of the following ions will be
Q47: Which of the following pairs of ions
Q66: A solution of ethanol (C<sub>2</sub>H<sub>6</sub>O) in water
Q95: All solutions are mixtures