Multiple Choice
Which of the following statements concerning a face-centered cubic unit cell and the corresponding lattice, made up of identical atoms, is incorrect?
A) The coordination number of the atoms in the lattice is 8.
B) The packing in this lattice is more efficient than for a body-centered cubic system.
C) If the atoms have radius r, then the length of the cube edge is 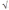 8 × r.
8 × r.
D) There are four atoms per unit cell in this type of packing.
E) The packing efficiency in this lattice and hexagonal close packing are the same.
Correct Answer:

Verified
Correct Answer:
Verified
Q21: A certain solid metallic element has a
Q36: The meniscus of mercury in a glass
Q39: A temperature increase causes _ in the
Q40: Which of the following atoms should have
Q47: Liquid sodium can be used as a
Q58: Which one of the following statements about
Q60: The strongest intermolecular interactions between ethyl alcohol
Q73: Ceramic superconductors often contain copper in unusual
Q76: Lead crystallizes in the face-centered cubic lattice.
Q90: A temperature increase causes _ in the