Multiple Choice
Which of the following statements relating to molecular orbital (MO) theory is incorrect?
A) Combination of two atomic orbitals produces one bonding and one antibonding MO.
B) A bonding MO is lower in energy than the two atomic orbitals from which it is formed.
C) Combination of two 2p orbitals may result in either σ or 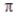 MOs.
MOs.
D) A species with a bond order of zero will not be stable.
E) In a stable molecule having an even number of electrons, all electrons must be paired.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: According to the molecular orbital (MO) treatment
Q8: A molecule with the formula AX<sub>3</sub> uses
Q10: According to molecular orbital theory, all diatomic
Q11: A carbon-carbon double bond in a molecule
Q15: Which one, if any, of the following
Q21: Valence bond theory predicts that tin will
Q30: In molecular orbital theory, molecules with an
Q31: One can safely assume that the 3s-
Q32: For which one of the following molecules
Q36: According to valence bond theory, which of