Multiple Choice
Oxygen difluoride is a powerful oxidizing and fluorinating agent. Select its Lewis structure.
A) 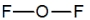
B) 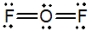
C) 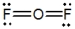
D) 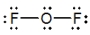
E) None of these choices are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Which one of the following molecules contains
Q17: The molecule AX<sub>2</sub>, where A and X
Q27: What is the molecular shape of BeH2
Q28: Select the correct Lewis structure for nitrogen
Q31: What is the molecular symmetry around the
Q33: In which one of the following is
Q35: According to VSEPR theory, a molecule with
Q36: Predict the ideal bond angles around nitrogen
Q40: Which one of the following molecules and
Q57: In formaldehyde, CH<sub>2</sub>O, both the formal charge