Multiple Choice
In the following Lewis structure for ClO3F, chlorine has a formal charge of ____ and an oxidation number of ____. 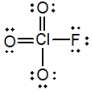
A) 7; 7
B) 7; -1
C) 1; 1
D) 1; -1
E) 1; 7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: In which one of the following species
Q18: How many resonance structures are possible for
Q21: Use VSEPR theory to decide which one
Q50: According to VSEPR theory, a molecule with
Q73: Predict the ideal bond angles in IF<sub>2</sub><sup>−</sup>
Q80: According to VSEPR theory, a molecule with
Q82: According to VSEPR theory, a molecule with
Q89: Select the correct Lewis structure for TeBr<sub>2</sub>.<br>A)
Q90: In carbon disulfide, how many lone pairs
Q97: Predict the smallest actual bond angle in