Multiple Choice
In the following Lewis structure for phosphate, phosphorus has a formal charge of ____ and an oxidation number of ____. 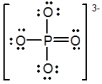
A) 0; -3
B) 0; 5
C) 5; -3
D) 5; 5
E) 3; 5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Predict the ideal bond angles in GeCl<sub>4
Q11: When resonance occurs, the bond lengths in
Q13: Which of the following molecules has a
Q31: According to VSEPR theory, a molecule with
Q54: In neutral molecules, how many bonds are
Q56: In which one of the following molecules
Q76: What is the molecular shape of ClF<sub>2</sub><sup>-</sup>
Q77: Which one of the following Lewis structures
Q82: What is the molecular shape of ClCN
Q89: The best Lewis structure for sulfuric acid