Multiple Choice
If astatine-207 decays by two α emission followed by an electron capture,what is the product nucleus? (Atomic number of astatine = 85)
A) 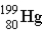
B) 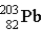
C) 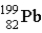
D) 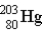
E) 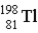
Correct Answer:

Verified
Correct Answer:
Verified
Q22: How many protons,electrons,and neutrons,respectively,does <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="How
Q23: Select the correct balanced reaction for the
Q24: What is the nuclear binding energy per
Q25: Which of the following elements does NOT
Q26: The half-life of carbon-14 is 5730 years.If
Q28: Write the symbol for calcium._
Q29: What is the most abundant element on
Q30: An atom with 45 protons has a
Q31: For the element sulfur, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="For
Q32: Hydrogen has four naturally occurring isotopes.