Multiple Choice
Calculate the energy released (per mole of deuterium consumed) for the following fusion reaction, 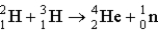 given the following molar masses of nucleons and nuclei.(c = 2.998 × 108 m/s)
given the following molar masses of nucleons and nuclei.(c = 2.998 × 108 m/s) 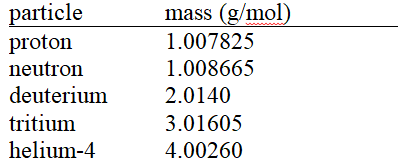
A) 5.63 × 106 J/mol
B) 1.69 × 1015 J/mol
C) 4.62 × 1013 J/mol
D) 8.44 × 1011 J/mol
E) 1.69 × 1012 J/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The symbol for the element iodine is<br>A)
Q2: Give the name and symbol for the
Q3: A 0.20-mL sample of a solution containing
Q4: Determine the number of protons and the
Q5: Write the name for K._
Q7: When <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="When undergoes
Q8: Determine the number of protons and the
Q9: Which of the following elements undergoes nuclear
Q11: How many neutrons are present in a
Q49: What is the percent activity of a