Multiple Choice
Which pair has approximately the same mass?
A) A hydrogen ( 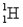 ) and a deuterium (
) and a deuterium ( 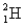 ) atom
) atom
B) A neutron and an electron
C) A proton and a neutron
D) An electron and a proton
E) A hydrogen ( 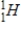 ) and a tritium (
) and a tritium ( 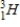 ) atom
) atom
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q167: If a nucleus emits an alpha particle,_.<br>A)
Q168: Complete the following fusion reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg"
Q169: How many atoms are represented by one
Q170: The number of neutrons in one atom
Q171: The rate constant for the decay of
Q173: The gold-197 nucleus has a binding energy
Q174: Which of the following is a noble
Q175: All isotopes with atomic number greater than
Q176: Which of the following is a metalloid?<br>A)
Q177: Choose the pair in which the components