Multiple Choice
Which of the following chemical equations depicts an alkylation reaction?
A) C6H6( 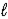 ) + CH3Cl(
) + CH3Cl( 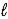 ) → C6H5CH3(
) → C6H5CH3( 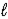 ) + HCl(g)
) + HCl(g)
B) 2 CH3OH( 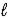 ) + 3 O2(g) → 2 CO2(g) + 4 H2O(
) + 3 O2(g) → 2 CO2(g) + 4 H2O( 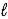 )
)
C) C6H12( 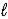 ) → C6H10(
) → C6H10( 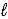 ) + H2(g)
) + H2(g)
D) CH2ClCH2Cl(g) + H2(g) → CH3CH3(g) + Cl2(g)
E) CHClCHCl(g) → CH2ClCH2Cl(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q61: CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>COOH is _.<br>A) isobutyl alcohol<br>B) butyl alcohol<br>C)
Q62: Which one of the following statements concerning
Q63: What is the classification of the molecule
Q65: An aldehyde may be produced by the
Q67: How many structures (i.e.,unique isomers)exist for tribromobenzene?<br>A)
Q68: What is the name of the product
Q69: Which of the following is the product
Q70: Which of the following alcohols is least
Q71: What is the name of the given
Q76: What is the molecular formula of a