Multiple Choice
In an octahedral complex,electrons in  and
and 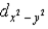 orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals
orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals
A) contain more electrons than the other three d orbitals.
B) each contain one unpaired electron.
C) are oriented directly toward the incoming ligand electron pairs.
D) are located a greater distance from the metal ion nucleus.
E) are roughly spherical in shape,much like an s orbital.
Correct Answer:

Verified
Correct Answer:
Verified
Q36: The calcium silicate formed in the blast
Q37: What is the name of the compound
Q38: The period 6 transition metals have greater
Q39: Which element has the highest density?<br>A) Zn<br>B)
Q40: Which of the following pairs of d-block
Q42: Which element has the following ground state
Q43: What is the coordination number of the
Q44: Ions such as [Co(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup> and [Ag(CN)<sub>2</sub>]<sup>-</sup> are
Q45: What is the maximum oxidation state expected
Q46: The iron produced in a blast furnace