Multiple Choice
Metal hydrides are used to remove traces of water from nonpolar solvents.Write a balanced chemical equation for the reaction of lithium hydride and water.
A) LiH2(s) + 2 H2O( 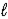 ) → Li(s) + 2 H3O+(aq)
) → Li(s) + 2 H3O+(aq)
B) 2 LiH(s) + 2 H2O( 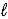 ) → 2 LiOH(s) + H2(g)
) → 2 LiOH(s) + H2(g)
C) 2 LiH(s) + H2O( 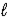 ) → Li2O(s) + 2 H2(g)
) → Li2O(s) + 2 H2(g)
D) 2 LiH(s) + H2O( 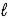 ) → Li2O(s) + 4 H+(aq)
) → Li2O(s) + 4 H+(aq)
E) LiH(s) + H2O( 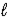 ) → Li(s) + H3O+(aq)
) → Li(s) + H3O+(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q49: One commercial method of producing Cl<sub>2</sub> is
Q50: Catalytic steam reformation of hydrocarbons (such as
Q51: Write a balanced chemical equation for the
Q52: What mass of CO<sub>2</sub> would be produced
Q53: Nitric acid decomposes slowly in sunlight with
Q55: Molten Al<sub>2</sub>O<sub>3</sub> is reduced by electrolysis at
Q56: What mass of MgO would be produced
Q57: Which group 1A metal is the strongest
Q58: Aluminum is resistant to corrosion because:<br>A) its
Q59: All of the following statements concerning sodium