Multiple Choice
Write a balanced chemical equation for the reaction of potassium and water.
A) K(s) + H2O( 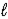 ) → KO(s) + H2(g)
) → KO(s) + H2(g)
B) 2 K(s) + 2 H2O( 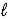 ) → 2 KOH(aq) + H2(g)
) → 2 KOH(aq) + H2(g)
C) 2 K(s) + 2 H2O( 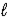 ) → K2O2(s) + 2 H2(g)
) → K2O2(s) + 2 H2(g)
D) 2 K(s) + H2O( 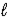 ) → K2O(s) + H2(g)
) → K2O(s) + H2(g)
E) 4 K(s) + 2 H2O( 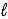 ) → 4 KH(s) + O2(g)
) → 4 KH(s) + O2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Predict the formula of the product resulting
Q32: Which of the following is the most
Q33: Give two examples of the allotropes of
Q34: Which of the following statements is INCORRECT?<br>A)
Q35: The products of the reaction of strontium
Q37: Silicon is purified in a process called
Q38: Quartz is a pure crystalline form of
Q39: The only acid that decomposes silicon dioxide
Q40: Which of the following elements does not
Q41: The primary source material for the commercial