Multiple Choice
Write a balanced chemical equation for the reaction of lime and water to form slaked lime.
A) CaO(s) + 2 H2O( 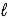 ) → CaCO3(s) + 2 H2(g)
) → CaCO3(s) + 2 H2(g)
B) CaO(s) + H2O( 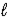 ) ⇋ Ca(OH) 2(s)
) ⇋ Ca(OH) 2(s)
C) Ca3(PO4) 2(s) + 2 H2O( 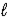 ) → 2 CaHPO4(aq) + Ca(OH) 2(aq)
) → 2 CaHPO4(aq) + Ca(OH) 2(aq)
D) CaCO3(s) + H2O( 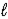 ) ⇋ Ca(s) + H3O+(aq) + CO2(g)
) ⇋ Ca(s) + H3O+(aq) + CO2(g)
E) CaCO3(s) + H2O( 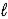 ) → CaO(s) + H2CO3(aq)
) → CaO(s) + H2CO3(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q70: Which of the following is not a
Q71: The active ingredients in "strike anywhere" matches
Q72: A class of aluminosilicate,called a(n)_,contains regularly spaced
Q73: Which of the following statements is INCORRECT?<br>A)
Q74: The primary product from the reaction of
Q76: Which of the following balanced chemical equations
Q77: Dinitrogen monoxide (laughing gas)can be produced by
Q78: Which list contains the three most abundant
Q79: Ammonium perchlorate can decompose violently according to
Q80: Which of the following calcium compounds is