Multiple Choice
Based on the following data,what is the I-I bond energy? 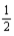 H2(g) +
H2(g) + 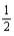 I2(g) → HI(g) ; ΔrH° = 26.36 kJ/mol-rxn Bond
I2(g) → HI(g) ; ΔrH° = 26.36 kJ/mol-rxn Bond
Bond Energy (kJ/mol)
H-H
435
H-I
295
A) 365 kJ/mol
B) 208 kJ/mol
C) -208 kJ/mol
D) -155 kJ/mol
E) 155 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: What is the oxidation state of each
Q11: Which two elements are most abundant in
Q12: Arrange the following in order of increasing
Q13: Which of the following nitrogen oxides are
Q14: Which of the following reactions does not
Q16: What is the total number of valence
Q17: What is the molecular formula of chlorous
Q18: Which of the following does not contain
Q19: Which of the following methods is used
Q20: What is the highest oxidation state possible