Multiple Choice
An SHE electrode has been assigned a standard reduction potential,E°,of 0.00 volts.Which of the following reactions will occur at this electrode?
A) 2 H2O( 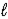 ) + 2 e- → H2(g) + 2 OH-(aq)
) + 2 e- → H2(g) + 2 OH-(aq)
B) O2(g) + 4 e- → 2 O2-(aq)
C) Hg2Cl2(s) + 2 e- → 2 Hg( 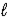 ) + 2 Cl-(aq)
) + 2 Cl-(aq)
D) Li+(aq) + e- → Li(s)
E) 2 H+(aq) + 2 e- → H2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q29: The following electrochemical cell has a potential
Q30: In the context of the diagram given
Q31: Which of the following statements concerning voltaic
Q32: When the given oxidation-reduction reaction in an
Q33: Which of the following statements is true
Q35: Write the balanced reduction half-reaction for the
Q36: For the electrochemical cell Cu(s)| Cu<sup>2+</sup> ||
Q37: The following has a potential of 0.34
Q38: Write a balanced half-reaction for the reduction
Q39: Aluminum(III)ion (Al<sup>3+</sup>)is reduced to solid aluminum at